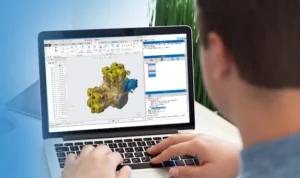
Medical Device Manufacturing Industry DFM Software – DFMPro
Rising medical device recalls and cost and public nature of these events will force medical device manufacturers to focus on quality and reliability throughout product design and-manufacturing
Rising Product Recalls
- Increasing product recalls call for additional measures from OEMs to ensure they are at par with their competition. Medical device manufacturers must warrant top notch product quality to avoid product recalls.
- Medical device manufacturers are experiencing revenue declines due to delays in time-to-market, as well as lost sales and market share, reputational capital and brand equity losses.
Increasing Product Complexity
- Medical devices are becoming more and more complex due to inclusions of software & innovative technologies
- As medical devices become more and more connected to the internet, smartphones, electronic health data, and facility networks, they become more vulnerable
Stringent Compliance Standards
- Complying with Unique Device Identification (UDI) and EU Medical Device Regulations (MDR) is another stressor for medical device manufacturing companies at an already challenging time, potentially affecting both top and bottom-line growth
- The increased complexity and volume of medical device marketing submissions has increased the time taken by the FDA approval by 55%
How DFMPro Can Help
Get to Market Quickly with High Quality, Innovative Products Delivered at a Competitive Cost
With DFMPro software solutions, Medical device manufacturers can focus on getting to market quickly with high quality, innovative products delivered at a competitive cost
Develop High Quality Medical Devices Faster
- Designing first time right, avoid downstream manufacturability issues and rework
- Quickly validate quality & safety requirements / standards
- Improve Time-to-Market
Be efficient in R&D and Product Development to Acclimatize Changing Regulatory Environment and Bringing in Innovation
- Knowledge capture & standardization at organizational level resulting in continuous process improvement and reuse
- Scalable framework for capturing and disseminating manufacturing knowledge upstream
Improve collaboration with global suppliers and Operational Efficiency
- Capture supplier capabilities and Improve design collaboration between departments, suppliers and vendors
Our Customers
Resources
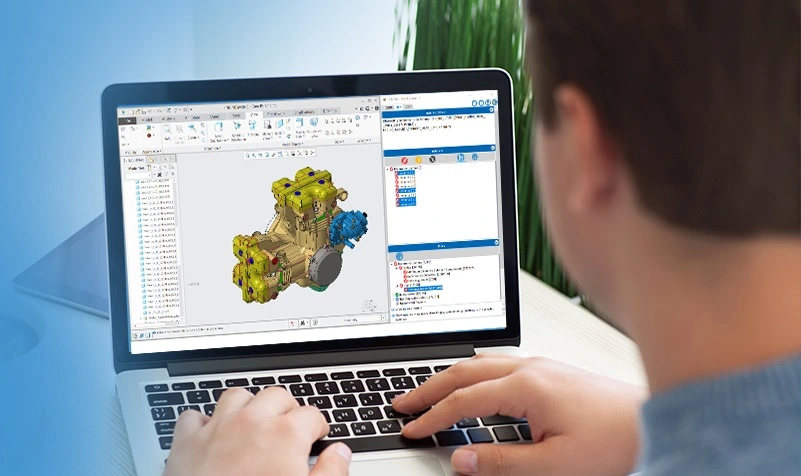
Designing for Manufacturability: How Alcon saves time and reduces cost with Creo and DFMPro
Learn from Alcon a pioneering medical manufacturer of eye care components on how they have achieved early visibility into the manufacturability and could save time to market and reduce the product cost with DFMPro and Creo.

How to enhance productivity and maximize returns with DFMPro for NXTM
Learn how DFMPro facilitates continuous process improvement through knowledge capture and reuse and how to tackle today’s challenges of faster time to market with optimal cost and high quality product.

How two manufacturing companies implemented design for manufacturing software and what they have learned
In this webinar, Joe Barkai will explore the benefits of manufacturing verification tools as experienced by two manufacturers of highly complex equipment from the medical imaging and semiconductor fabrication industries.
Why and How of DFM Review Automation
A DFM review automation tool helps in identifying areas in a design that are difficult, expensive or impossible to manufacture. It eases and reduces errors in the design validation process. Learn more in this whitepaper.